
CYRAMZA can help fight your advanced or metastatic stomach or GE junction cancer
CYRAMZA is used by itself or with a chemotherapy medicine called paclitaxel to treat certain kinds of stomach cancer or cancer of the area where the stomach and esophagus (food pipe) meet that is advanced or has spread to other parts of the body. The area where the stomach and esophagus meet is often called the gastroesophageal (GE) junction. CYRAMZA is for people whose stomach cancer got worse during or after certain other types of chemotherapy.
SELECT SAFETY INFORMATION
CYRAMZA may cause serious side effects, including:
Tears in the stomach or bowel wall may happen with CYRAMZA. This can be life threatening. Tell your doctor if you have severe diarrhea, vomiting, or severe abdominal pain. If you have tears in the stomach or bowel wall, you will have to stop receiving CYRAMZA.
Nearly 2x as many people had tumor shrinkage
In a clinical study, nearly 2x as many people had their tumors shrink with CYRAMZA + paclitaxel (a type of chemotherapy) vs chemotherapy alone
- Tumors shrank (by 30% or more) in 28% of people taking CYRAMZA + chemotherapy vs 16% taking chemotherapy alone

Slowed the progression of disease
CYRAMZA + chemotherapy delayed disease progression for longer than chemotherapy alone
- Half of the people who received CYRAMZA + chemotherapy lived without their cancer getting worse for 4.4 months vs 2.9 months with chemotherapy alone

May help you live longer
People who received CYRAMZA + chemotherapy had a longer life than those who received chemotherapy alone
- Half of the people who received CYRAMZA + chemotherapy after their cancer progressed were still alive at 9.6 months vs 7.4 months with chemotherapy alone
A clinical study of 665 people with advanced or metastatic stomach cancer or gastroesophageal (GE) junction cancer who received CYRAMZA + paclitaxel (330 people) vs those who received paclitaxel alone (335 people). All people in the trial had previously received fluoropyrimidine- and platinum-containing chemotherapy.
SELECT SAFETY INFORMATION
CYRAMZA may cause serious side effects, including:
Wounds may not heal quickly or completely. Tell your doctor if you have a wound that doesn’t heal properly or have a surgery planned. If you are having surgery, CYRAMZA treatment should be stopped beforehand. Your doctor may put you back on CYRAMZA after your surgical wound has healed.
Explore the Lilly Oncology Support Center for information on savings and financial assistance.
What to expect during treatment
You've decided to move forward and are ready to start treatment.

Treatment will be given by an intravenous (IV) infusion, in either the doctor's office, a hospital, or an infusion center.

CYRAMZA will be given by an intravenous (IV) infusion, in either the doctor's office, a hospital, or an infusion center. CYRAMZA is given alone or in combination with a type of chemotherapy, paclitaxel.
CYRAMZA will take about 60 minutes to administer. If taken with chemotherapy, CYRAMZA will be given first (60-minute infusion), followed by your chemotherapy (60-minute infusion). If you handle the first infusion of Cyramza well, then your next Cyramza infusions may take only 30 minutes. Your doctor will determine the number of treatments you receive.
Before you receive CYRAMZA, your doctor will give you different medicines to help prevent an allergic reaction that may occur during the infusion.

CYRAMZA will be given alone or in combination with paclitaxel (a type of chemotherapy).
SELECT SAFETY INFORMATION
CYRAMZA may cause serious side effects, including:
Strokes, mini-strokes, blood clots, and heart attacks have happened to people on CYRAMZA. These can be fatal. If you have one of these events, you will have to stop receiving CYRAMZA.
Understand your dosing schedule with CYRAMZA
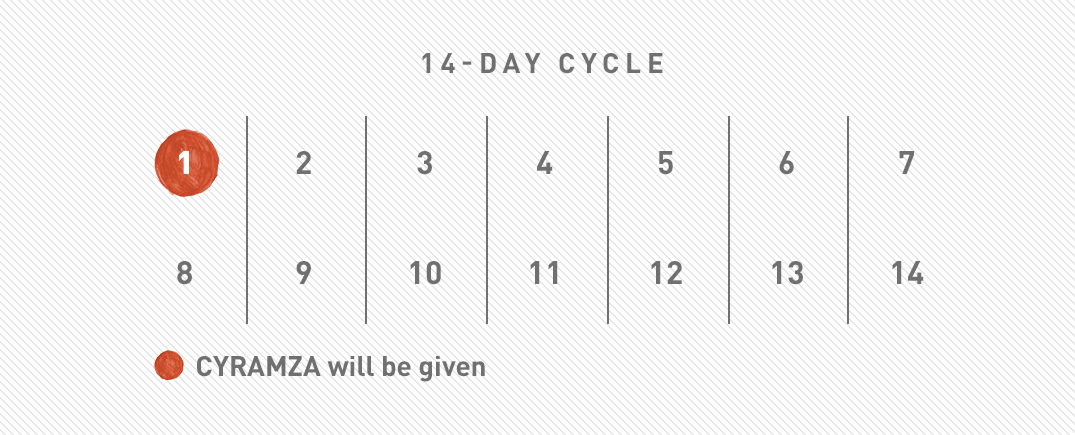
CYRAMZA alone
CYRAMZA will be given to you once every 2 weeks, or as recommended by your doctor.
Your doctor will determine the number of treatments you receive.
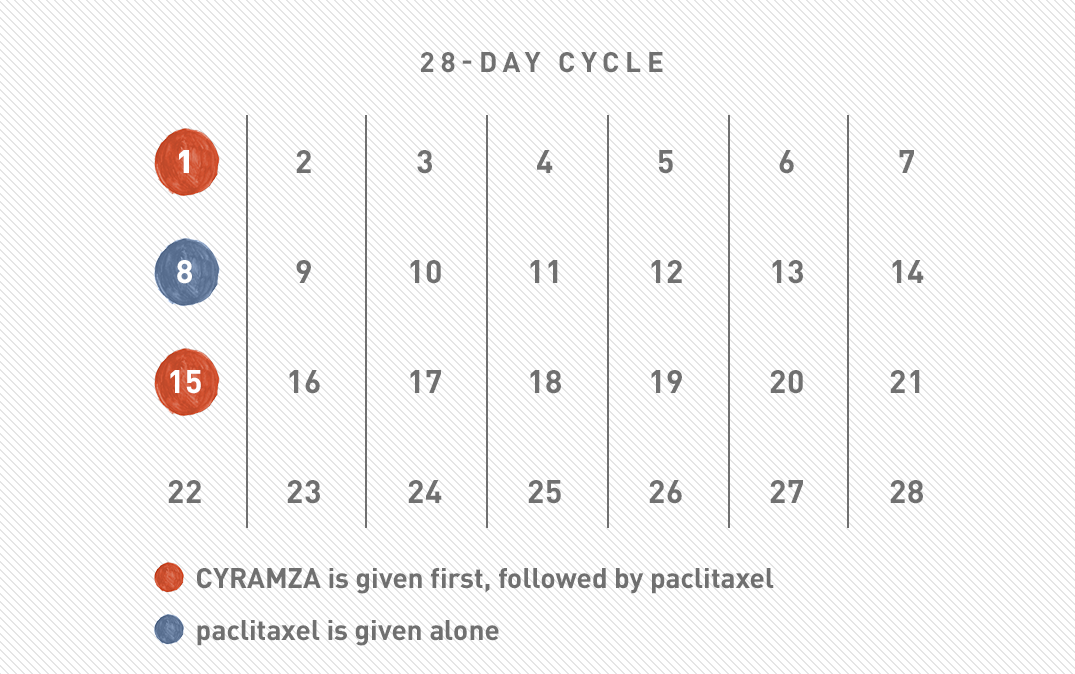
Gastric cancer 28-day calendar
CYRAMZA + PACLITAXEL (A TYPE OF CHEMOTHERAPY)
Weeks 1 and 3: CYRAMZA + paclitaxel will be given. CYRAMZA will be given first, followed by paclitaxel.
Week 2: Paclitaxel will be given alone.
Week 4: You can take the week off—it’s the “no treatment” week.
Your doctor will determine the number of treatments you receive.
SELECT SAFETY INFORMATION
CYRAMZA may cause serious side effects, including:
Reactions related to infusing CYRAMZA have happened. These can be severe and life threatening. Most of these reactions happened during or after the first or second CYRAMZA infusion. Symptoms of infusion reactions include shaking or stiffness of the body, back pain or spasms, chest pain or tightness, chills, flushing (sudden warmth and/or reddened skin on the face, neck, or upper chest), difficulty breathing, wheezing (a whistling sound in the breath caused by narrowed breathing tubes), becoming blue due to lack of oxygen, and tingling or numbness of the skin. In severe reactions, rapid heartbeat, low blood pressure, and severe trouble breathing may happen. Your health care team will give you medicine before each CYRAMZA infusion and will watch you for these side effects. If a reaction happens, CYRAMZA treatment may be infused at a slower rate or may be permanently stopped, depending on how severe the reaction is.
Tips for day of treatment and beyond
You may be nervous about your first treatment. Or maybe you're an old pro at this. But no matter where you're coming from, here are some important tips to help get you through each infusion with CYRAMZA:
Eating healthy and living well
When living with stomach cancer or GE junction cancer, good nutrition is essential. Talk to your doctor about strategies that can help keep you eating healthy. He or she might recommend eating multiple meals a day to help you maintain weight and balance your blood sugar. Your doctor might also mention incorporating more high-protein foods, like:
Fish

Lean meats

Cooked Vegetables

Smoothies
However, maintaining a nutritious diet can be difficult if you’re too tired to eat or having trouble keeping anything down. You may be experiencing side effects from treatment, like nausea or mouth sores, that also may cause you to lose your appetite.
You may be feeling frustrated. It can be hard to retrain your body to eat in this new way. But there are resources available that can help, including simple, nutritious recipes that are designed for people living with stomach cancer or GE junction cancer.
In addition to eating right, it’s important to keep balance in your life. Reducing stress can help you feel energized, so you can focus on the things that matter most. Stress-reducing activities include:
Exercise

Resting

Yoga or meditation
