
This is a patient with rapidly progressing mCRC*. Someone whose disease progressed within 6 months on first-line treatment with bevacizumab + mFOLFOX6.
Someone who is aware of their situation, but is not surrendering to it.
- Diagnosed with tumor in distal colon and multiple liver metastases
- ECOG PS 1
- KRAS exon 2 mutation positive
- Developed additional metastases in liver and right lung
Can you picture one of your patients?. Someone who, in the face of adversity, remains determined? Someone who is ready for what’s next?
* Hypothetical patient example.
ECOG=Eastern Cooperative Oncology Group;
Metastatic Colorectal Cancer
CYRAMZA, in combination with FOLFIRI (irinotecan, folinic acid, and fluorouracil), is indicated for the treatment of patients with metastatic colorectal cancer (mCRC) with disease progression on or after prior therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine.
SELECT IMPORTANT SAFETY INFORMATION
Hemorrhage
- CYRAMZA increased the risk of hemorrhage and gastrointestinal hemorrhage, including Grade ≥3 hemorrhagic events. In 2137 patients with various cancers treated with CYRAMZA, the incidence of all Grade hemorrhage ranged from 13-55%. Grade 3-5 hemorrhage incidence ranged from 2-5%.
- Permanently discontinue CYRAMZA in patients who experience severe (Grade 3 or 4) bleeding.
For your patients whose mCRC has progressed
What would you do at disease progression in mCRC?1

For your patients whose metastatic colorectal cancer (mCRC) has progressed, consider a switch to CYRAMZA plus irinotecan, folinic acid, and fluorouracil (FOLFIRI).
What would you do at disease progression in mCRC?
If patient's disease progressed on bevacizumab plus folinic acid, fluorouracil, and oxaliplatin (FOLFOX), how do you proceed with patients who have rapidly progressing disease? What are your options for patients with KRAS mutant disease? What would be your next step for this patient be? This is a hypothetical patient example.
KRAS mutation status was a prespecified subgroup in the RAISE trial. Any analyses of prespecified subgroups are exploratory.
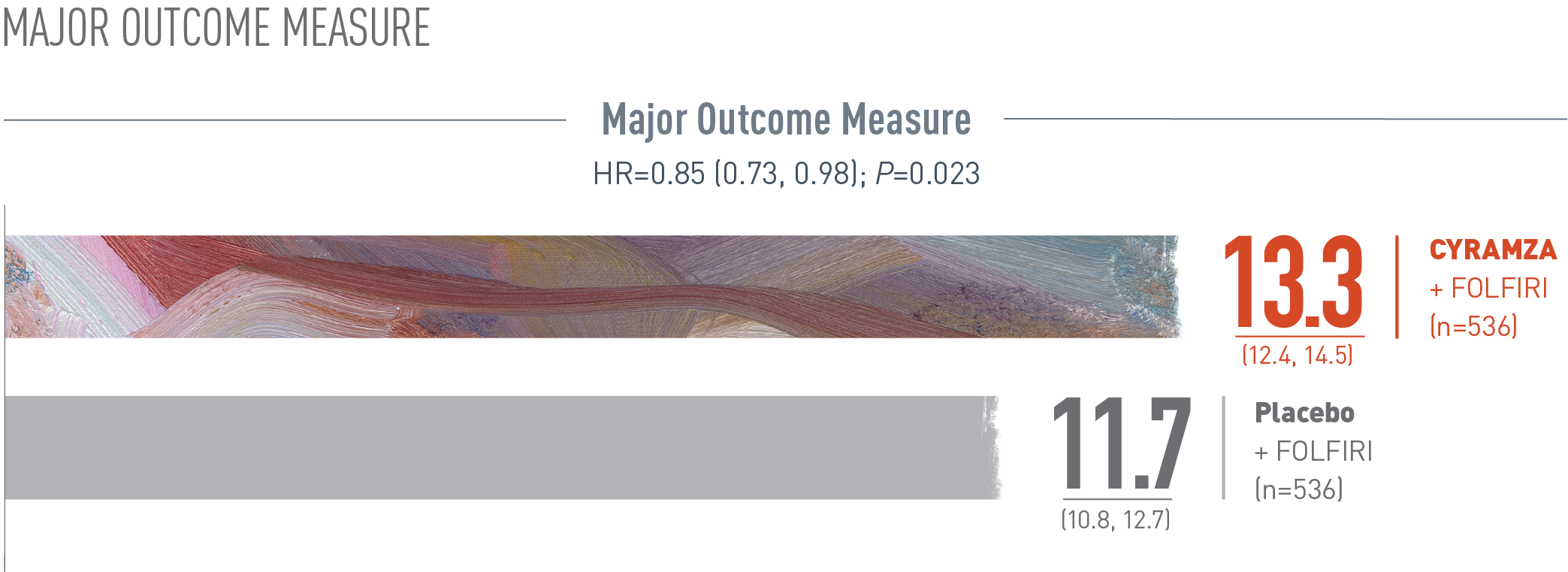
CYRAMZA + FOLFIRI demonstrated a statistically significant improvement in OS1 with consistent results in patients regardless of time to disease progression or KRAS status1-3
RAISE OS: Median—Months (95% CI)
The following results for patients with metastatic colorectal cancer (mCRC) in the RAISE trial are presented.
Overall survival (OS) was the major efficacy outcome measure of the RAISE trial. With CYRAMZA plus irinotecan, folinic acid, and fluorouracil (FOLFIRI), (n equals 536), median OS was 13.3 months with a 95 percent confidence interval of 12.4 to 14.5 months. With placebo plus FOLFIRI (n equals 536) median OS was 11.7 months with a 95 percent confidence interval of 10.8 to 12.7 months. The hazard ratio was 0.85 with a 95 percent confidence interval of 0.73 to 0.98 and the P value equaled 0.023.
The percentage of deaths at the time of analysis was 69% (372 patients) and 74% (397 patients) in the CYRAMZA + FOLFIRI and placebo + FOLFIRI arms, respectively1
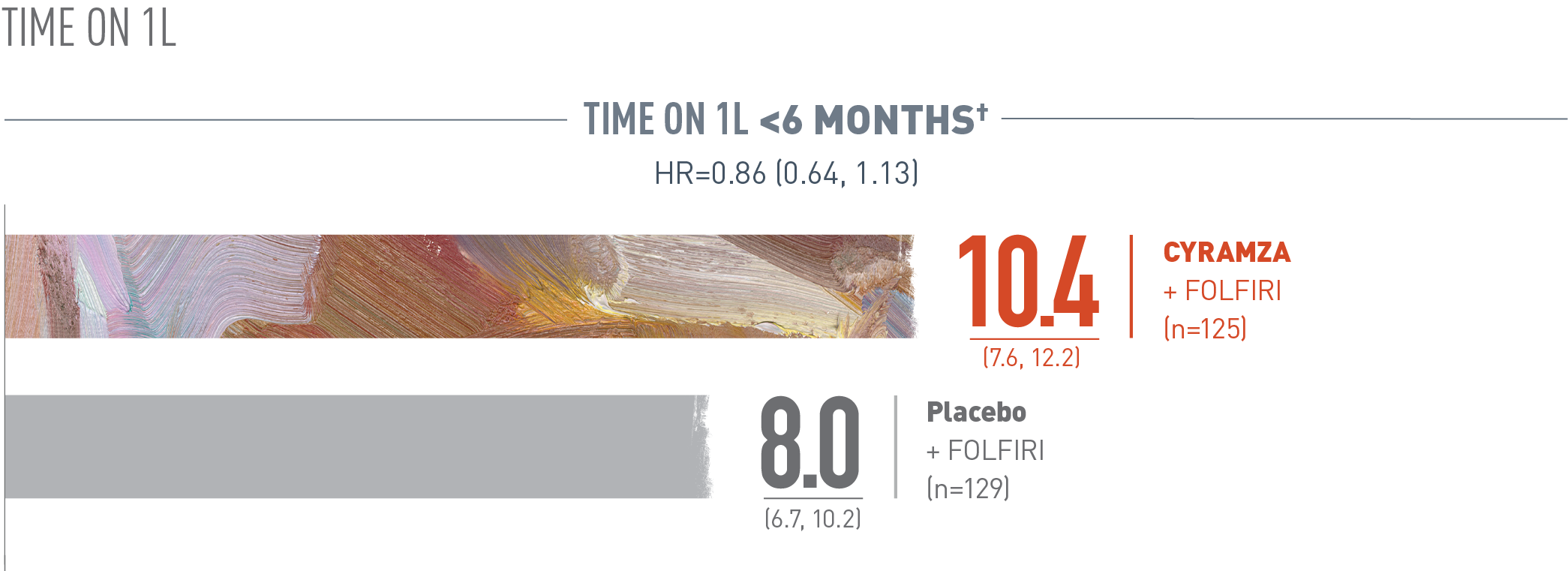
Overall survival (OS) was assessed in a subgroup of patients whose time on first-line (1L) treatment was less than six months. The following results are presented. With CYRAMZA plus irinotecan, folinic acid, and fluorouracil (FOLFIRI), (n equals 125), median OS was 10.4 months with a 95 percent confidence interval of 7.6 to 12.2 months. With placebo plus FOLFIRI (n equals 129) median OS was 8.0 months with a 95 percent confidence interval of 6.7 to 10.2 months. The hazard ratio was 0.86 with a 95 percent confidence interval of 0.64 to 1.13.
Time on 1L refers to disease progression on first-line therapy. Based on case report form (CRF) data if present, or interactive voice response system value if CRF data were missing for the parameter.
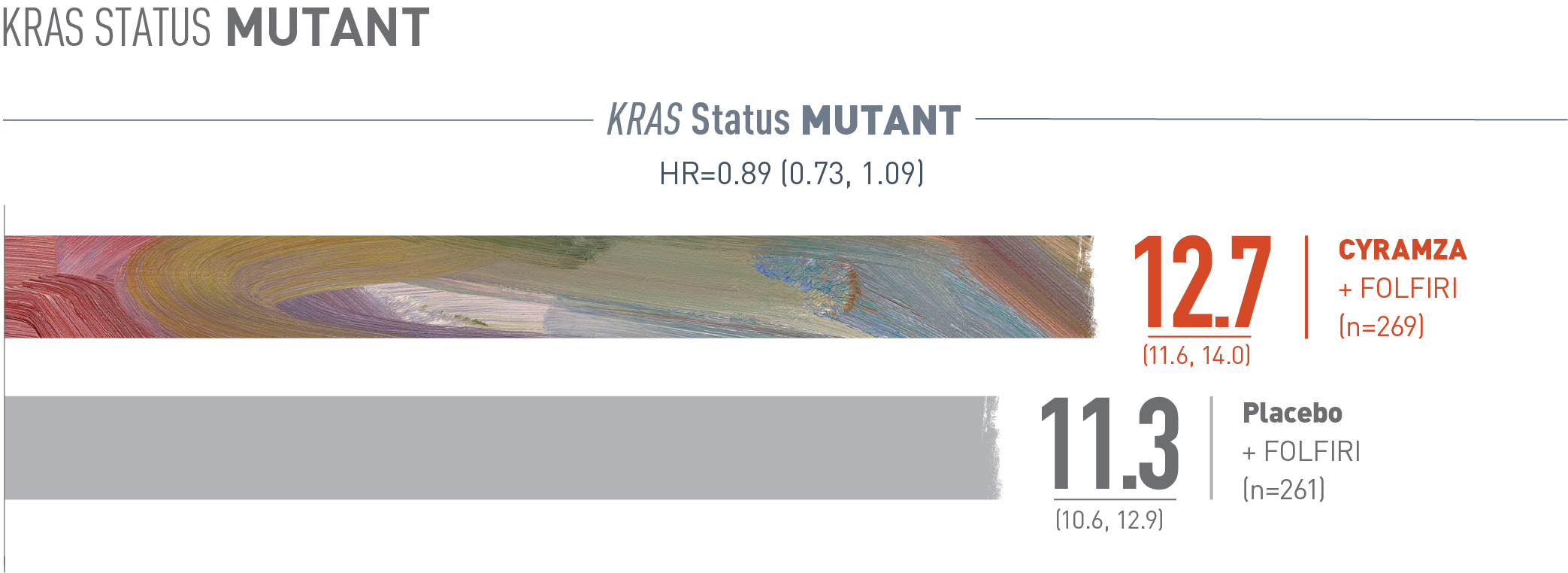
Overall survival (OS) was assessed in a subgroup of patients whose tumors carry a Kirsten rat sarcoma (Kras) mutation. The following results are presented. With CYRAMZA plus irinotecan, folinic acid, and fluorouracil (FOLFIRI), (n equals 269), median OS was 12.7 months with a 95 percent confidence interval of 11.6 to 14.0 months. With placebo plus FOLFIRI (n equals 261) median OS was 11.3 months with a 95 percent confidence interval of 10.6 to 12.9 months. The hazard ratio was 0.89 with a 95 percent confidence interval of 0.73 to 1.09.
The RAISE trial was not adequately powered, nor error-controlled, for subgroup analyses. Treatment differences observed in these subgroups cannot be regarded as statistically significant. The analyses described here were pre-specified and exploratory.
- Patients with time to disease progression on 1L ≥6 months (HR=0.86 [95% CI: 0.73, 1.01]): Median OS: 14.3 months with CYRAMZA + FOLFIRI (13.1, 15.5), n=411, vs 12.5 months with placebo + FOLFIRI (11.7, 13.6), n=407‡
- Patients with wild-type KRAS (HR=0.82 [95% CI: 0.67, 1.00]): Median OS: 14.4 months with CYRAMZA + FOLFIRI (12.7, 16.1), n=267, vs 11.9 months with placebo + FOLFIRI (10.8, 13.3), n=275§
† Time on 1L refers to disease progression on first-line therapy. Based on case report form (CRF) data if present, or interactive voice response system value if CRF data were missing for the parameter.
‡ For patients with 1L progression <6 months, the percentage of deaths at the time of analysis in the CYRAMZA + FOLFIRI arm was 73.6% (92 patients) and 79.1% (102 patients) in the placebo + FOLFIRI arm. For patients with 1L progression ≥6 months, the percentage of deaths at the time of analysis in the CYRAMZA + FOLFIRI arm was 68.1% (280 patients) and 72.5% (295 patients) in the placebo + FOLFIRI arm.3
§ For KRAS mutant patients, the percentage of deaths at the time of analysis in the CYRAMZA + FOLFIRI arm was 72.9% (196 patients) and 75.9% (198 patients) in the placebo + FOLFIRI arm. For KRAS wild-type patients, the percentage of deaths at the time of analysis in the CYRAMZA + FOLFIRI arm was 65.9% (176 patients) and 72.4% (199 patients) in the placebo + FOLFIRI arm.3
Supportive outcome measure:
Median PFS was 5.7 months with CYRAMZA + FOLFIRI1
(95% CI: 5.5, 6.2) (n=536) vs 4.5 months with placebo + FOLFIRI (95% CI: 4.2, 5.4) (n=536) (HR=0.79 [95% CI: 0.70, 0.90]); P<0.001
- The percentage of events at the time of analysis was 89% (476 patients) and 92% (494 patients) in the CYRAMZA + FOLFIRI and placebo + FOLFIRI arms, respectively1
- 73 of 476 events in CYRAMZA-treated patients and 64 of 494 events in placebo-treated patients were deaths.
SELECT IMPORTANT SAFETY INFORMATION
The labeling for CYRAMZA contains warnings and precautions for hemorrhage and GI hemorrhage, including severe and sometimes fatal events; gastrointestinal (GI) perforations, a potentially fatal event; impaired wound healing; arterial thromboembolic events (ATEs), including serious and sometimes fatal events; hypertension; infusion-related reactions (IRR), including severe and sometimes fatal events; worsening of pre-existing hepatic impairment; posterior reversible encephalopathy syndrome (PRES), including fatal events; proteinuria including nephrotic syndrome; thyroid dysfunction; and embryo-fetal toxicity. CYRAMZA should be permanently discontinued in patients who experience severe bleeding, a GI perforation, an ATE, uncontrolled hypertension, severe IRR, PRES, or urine protein >3 grams/24 h or nephrotic syndrome.
In RAISE, the most common adverse reactions (all Grades) observed in CYRAMZA with FOLFIRI-treated patients at a rate of ≥30% and ≥2% higher than placebo with FOLFIRI were diarrhea (60% vs 51%), neutropenia (59% vs 46%), decreased appetite (37% vs 27%), epistaxis (33% vs 15%), and stomatitis (31% vs 21%). The most common serious adverse reactions with CYRAMZA with FOLFIRI were diarrhea (3.6%), intestinal obstruction (3.0%), and febrile neutropenia (2.8%); 20% of patients treated with CYRAMZA with FOLFIRI received granulocyte colony-stimulating factors.
RAISE Trial Design (N=1072)
The phase III RAISE trial evaluated the efficacy and safety of CYRAMZA plus FOLFIRI vs placebo plus FOLFIRI in patients with mCRC with disease progression on or after prior therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine. Major efficacy outcome measure was OS. Supportive efficacy outcome measure was PFS. All patients were required to have ECOG PS 0 or 1. Patients were stratified by geographic region, KRAS mutation status, and time to disease progression after the beginning of first-line treatment (<6 months vs ≥6 months). Patients were randomized 1:1 to receive either CYRAMZA 8 mg/kg (n=536) or placebo (n=536), in combination with FOLFIRI, every 14 days.1
1L=first line; CI=confidence interval; ECOG=Eastern Cooperative Oncology Group; FOLFIRI=irinotecan, folinic acid, and fluorouracil; FOLFOX=folinic acid, fluorouracil, and oxaliplatin; HR=hazard ratio; ITT=intent-to-treat; KRAS=Kirsten rat sarcoma; mCRC=metastatic colorectal cancer; OS=overall survival; PFS=progression-free survival; PS=performance status.
References
- CYRAMZA (ramucirumab) package insert. Indianapolis, IN: Eli Lilly and Company; 2021.
- Obermannová R, Van Cutsem E, Yoshino T, et al. Subgroup analysis in RAISE: a randomized, double-blind phase III study of irinotecan, folinic acid, and fluorouracil (FOLFIRI) plus ramucirumab or placebo in patients with metastatic colorectal carcinoma progression. Ann Oncol. 2016;27(11):2082-2090.
- Data on File, Eli Lilly and Company. ONC20170425a.